Hatch breakout analysis
A hatch breakout analysis involves opening unhatched eggs (Dead-in-Shell) to determine at what stage of incubation embryo mortality has occurred. A hatch breakout is a useful tool for solving hatch problems and looking for areas to improve hatch performance.
Procedure
For a breakout analysis, five sample setter or hatcher trays per breeder flock and hatch should be identified. The trays should be chosen from different positions within the incubator and the number of eggs per tray should be recorded.
At fertility testing, open all eggs removed from the sample trays as clear or early dead germ. Do not refill the trays after fertility testing.
During hatch take-off, the number of culls and cracked eggs on each sample tray should be counted and all unhatched eggs opened. The results should be recorded on a record sheet to form part of the interpretation. Mortality must be expressed as a percent of eggs incubated.
Record whether the hatch debris was clean or dirty, the poults were still hatching (wet poults still on tray) or if poults were very thin as this provides clues as to advanced or late hatch.
A suggested staging for embryos:
- Infertile – no sign of embryo development, clear albumen and yellow yolk with white dot.
- Died 1 – 2 days – presence of white disc > 1cm on yolk, albumen may be cloudy, no evidence of blood vessels.
- Died 3 – 9 days – first sign of blood vessels on yolk up to embryo with limbs and beak visible but no egg tooth present (white dot on end of beak).
- �Died 10 – 15 days – egg tooth visible until first feathers present.
- Died 16 – 24 days – feathers first present until the embryo starts to withdraw yolk sac and moves into hatching position (head under right wing).
- �Died 25 – 27 days – yolk sac retraction started and in hatching position up to the start of pipping.
- �Pipped dead – shell pipped but embryo died.
- �Pipped alive – shell pipped and embryo still alive. Note if the membranes and feathers are dry or wet. This indicates whether the embryo has been pipped for a long time but has been unable to emerge from the shell or is a late hatching embryo.
1. Record any malpositioned embryos:
- �Type I – head between legs (normal position before day 25).
- �Type II – head in small end of egg.
- �Type III – head under left wing.
- �Type IV – head pointing away from air cell (rare).
- �Type V – leg overhead.
- �Type VI – head on top of right wing.
2. Record any abnormalities.
3. Record any contamination.
4. Record any cracked eggs.
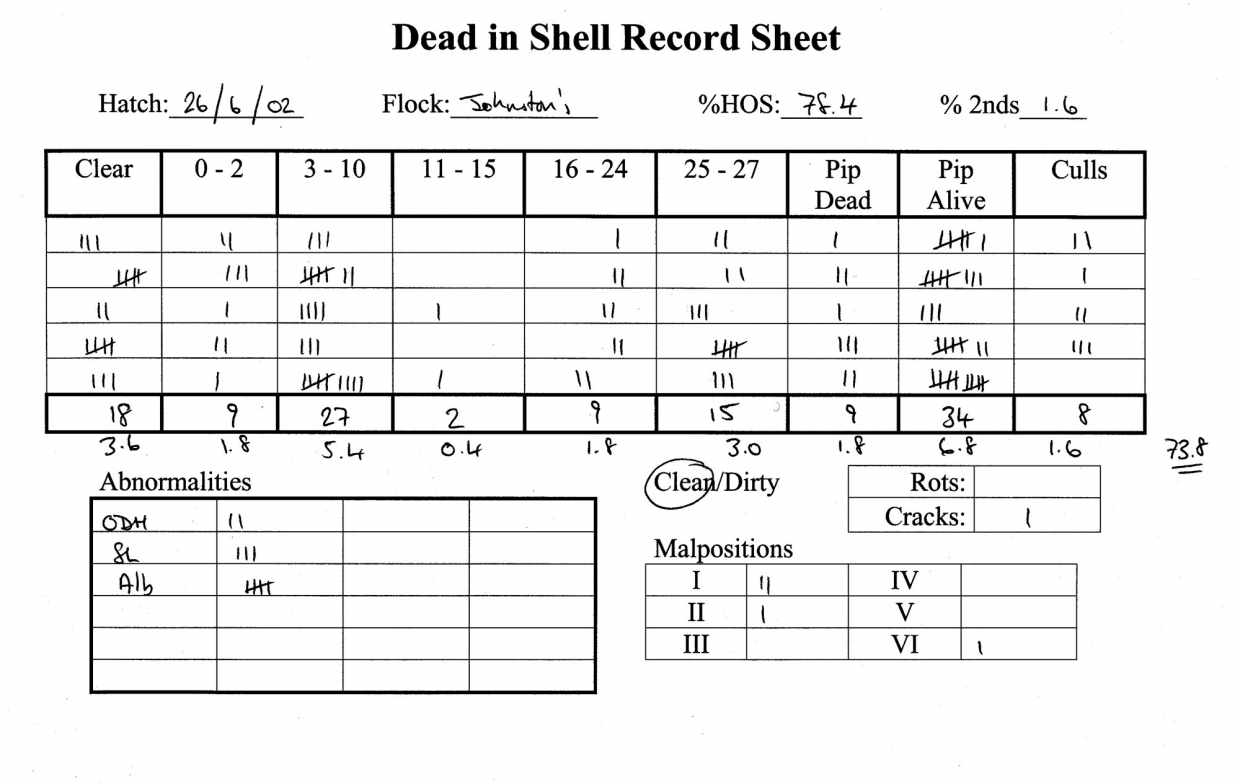
An example of a complete Dead in Shell Record Form is shown in Figure 1, each line represents one hatcher tray.
Interpretation of Results
Results from a hatch breakout should be combined with other information to provide a total picture. This information should include hatchability records, breeder performance records, incubation records and hatch timing information.
In a good hatching flock there are two main periods of embryo mortality: 3 – 10 days and 25 – 27 days + pips. High mortality at other development stages is not normal. A high incidence of mortality at a particular stage of development can indicate an acute problem during incubation caused by a machine failure.
A chronic problem, such as slight overheating, may result in mortality later in incubation.
Specific embryo abnormalities can be associated with specific problems (nutritional, incubation, toxins and disease) but it is important to note that the same abnormality can be the result of more than one problem. For example eye cataracts have been associated with high incubation temperature, mycotoxins and vitamin E deficiency.
Experience of hatch breakouts with good hatching flocks is important for understanding what is normal and abnormal.

